Electron Spin
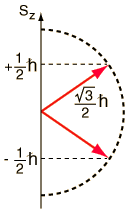 Spin "up" and "down" allows two electrons for each set of spatial quantum numbers.  | An electron spin s = 1/2 is an intrinsic property ofelectrons. Electrons have intrinsic angular momentumcharacterized by quantum number 1/2. In the pattern of other quantized angular momenta, this gives total angular momentum 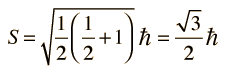 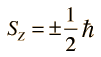 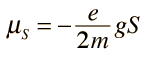 |




No comments:
Post a Comment